services
chromatography process development
-
affinity chromatography process development
-
ion exchange chromatography process development
-
hydrophobic chromatography process development
membrane filtration process development
-
harvest clarificationindepth filtration
-
nanofilm filtration
-
ultrafiltration/diafiltration
process transfer and scale-up
-
3 l/15 l process confirmation and transfer
-
200 l/500 l pilot production
advantages and capabilities
customized development services
express route
relying on accumulated industrial experience and the mature platform, a modular process development can be completed in 4-6 weeks for rapid submission.
stable route
relying on mature platform, chromatography process, membrane filtration process, virus inactivation and removal process could be developed within 6 ~ 10 weeks. accelerating development stage to scale-up pilot manufacturing and regulatory submission preparation.
refined development route
using scientific experimental methods such as doe, a 10-14 week immersive process development is finely tuned.
multiple 200-500 l pilot scale productions have been successfully completed.
leveraging a mature platform, multiple downstream process development for antibody drug projects has been completed, including products that have been approved for marketing or in the pre-bla, ind and pre-ind development stages.
demonstration case
objective: rapid development of a covid-19 antibody drug project
challenge: only preliminary small-scale process before the project was transferred, due to tight project timelines, there was a problem with long lead times for selected materials that could not be supplied for production.
solutions
-
process transferred in small-scale process, and project is mastered by team in quick.
-
materials were rapidly assessed, and alternative suppliers for affinity chromatography, cationic chromatography, and depth filtration were selected. with good relationships with suppliers, rapid stocking was achieved even when materials alternative materials have been determined.
-
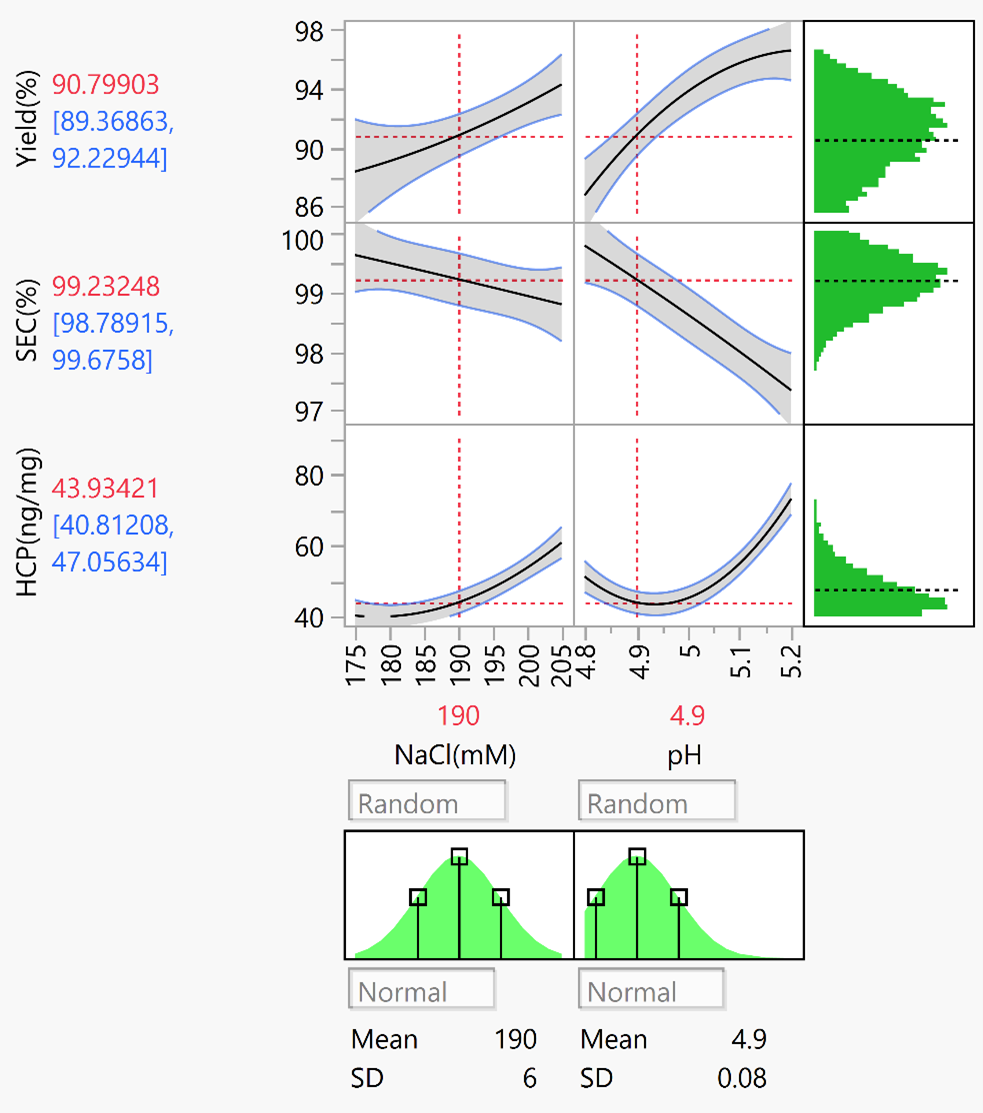
the doe scientific experimental design was adopted for process development. process parameters for project-specific affinity binding, washing, elution, and cation elution were established. quality data were compared in detail after changing materials, with coordinated timeline for new process development in quick.
-
during process development, development report were written and process transfer was carried out in parallel.
doe experimental results ( jmp software analysis)
project highlights
- customized solutions delivered ahead of schedule
- 20% reduction in production cost by changing process materials
workshop